Alzheimer’s disease (AD)
Alzheimer’s disease (AD)
Alzheimer's disease (AD), the most common cause of dementia, is a progressive neurodegenerative disorder with the characteristic of neuronal loss in cerebral cortex, hippocampus and amygdala. AD usually appears in older adults (late-onset); however, genetic mutations cause the rare early-onset AD. Although the knowledge about AD has increased in the past decades, an effective therapy is still lacking. More understanding the cellular and molecular mechanism will be helpful for the drug development for AD.
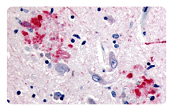
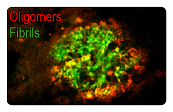
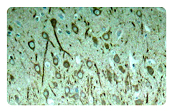
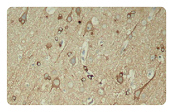
AD is characterized by amyloid plaques (senile plaques) and neurofibrillary tangles (NTFs). The main components of the amyloid plaques and neurofibrillary tangles are Aβ42 and hyperphosphorylated Tau, respectively. The extracellular Aβ oligomers trigger apoptosis and neurotoxicity through activation of death receptors and calcium channels; and the intracellular Aβ oligomers promote hyperphosphorylation of tau, mitochondria dysfunction, and ER stress, leading to neuronal loss.
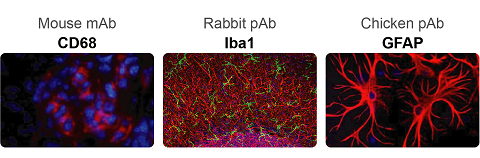
Neuroinflammation is another pathological feature of AD. Aβ fibrils activate microglial NFκB and NLRP3 inflammasome pathways, leading to pro-inflammatory cytokine release and the following neuroinflammation which manifests in microgliosis and astrogliosis. NLRP3 inflammasome is a complex that induces inflammation by triggering the maturation and release of pro-inflammatiory cytokine IL-1b and IL-18. Besides, NLRP3 activation also drive tau pathology by regulating the hyperphosphorylation of tau. Targeting NLRP3 inflammasome has emerged as a promising approach for AD treatment.
AD biomarkers play a critical role in early diagnosis of AD and prediction of disease progression. The cerebrospinal fluid (CSF) Aβ42, total tau (T-tau), and phosphorylated tau (P-tau) have been widely used as AD biomarkers as they reflect the key elements of AD pathophysiology. For example, reduced CSF Aβ42 and Aβ42/Aβ40 reflect brain amyloidosis; and increased CSF T-tau and P-tau reflect neurodegeneration.
In AD, toxic Aβ and P-tau can be carried and spread by exosomes which are able to pass through the BBB and be detected in the peripheral blood. These properties render exosomes as ideal biomarkers for AD. The levels of Aβ42, T-tau, p-T181 tau, and p-S396 tau provide a good predictor for disease development during the preclinical stage of AD.
|
▶ Human AD biomarkers |
|
▶ Rodent AD biomarkers |