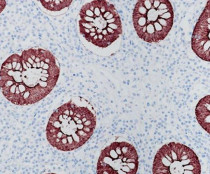
ARG66631
anti-Villin antibody [SQab19149]
anti-Villin antibody [SQab19149] for IHC-Formalin-fixed paraffin-embedded sections and Human
Overview
| Product Description | Recombinant Rabbit Monoclonal antibody [SQab19149] recognizes Villin |
|---|---|
| Tested Reactivity | Hu |
| Tested Application | IHC-P |
| Host | Rabbit |
| Clonality | Monoclonal |
| Clone | SQab19149 |
| Isotype | IgG |
| Target Name | Villin |
| Antigen Species | Human |
| Immunogen | Synthetic peptide within aa. 600-700 of Human Villin. |
| Conjugation | Un-conjugated |
| Alternate Names | Villin-1; D2S1471; VIL |
Application Instructions
| Application Suggestion |
|
||||
|---|---|---|---|---|---|
| Application Note | IHC-P: Antigen Retrieval: Heat mediation was performed in Tris/EDTA buffer (pH 9.0), primary antibody incubate at RT (18°C - 25°C) for 30 minutes. * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
Properties
| Form | Liquid |
|---|---|
| Purification | Purification with Protein A. |
| Buffer | PBS, 0.01% Sodium azide, 40% Glycerol and 0.05% BSA. |
| Preservative | 0.01% Sodium azide |
| Stabilizer | 40% Glycerol and 0.05% BSA |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | VIL1 |
| Gene Full Name | villin 1 |
| Background | This gene encodes a member of a family of calcium-regulated actin-binding proteins. This protein represents a dominant part of the brush border cytoskeleton which functions in the capping, severing, and bundling of actin filaments. Two mRNAs of 2.7 kb and 3.5 kb have been observed; they result from utilization of alternate poly-adenylation signals present in the terminal exon. [provided by RefSeq, Jul 2008] |
| Function | Epithelial cell-specific Ca(2+)-regulated actin-modifying protein that modulates the reorganization of microvillar actin filaments. Plays a role in the actin nucleation, actin filament bundle assembly, actin filament capping and severing. Binds phosphatidylinositol 4,5-bisphosphate (PIP2) and lysophosphatidic acid (LPA); binds LPA with higher affinity than PIP2. Binding to LPA increases its phosphorylation by SRC and inhibits all actin-modifying activities. Binding to PIP2 inhibits actin-capping and -severing activities but enhances actin-bundling activity. Regulates the intestinal epithelial cell morphology, cell invasion, cell migration and apoptosis. Protects against apoptosis induced by dextran sodium sulfate (DSS) in the gastrointestinal epithelium. Appears to regulate cell death by maintaining mitochondrial integrity. Enhances hepatocyte growth factor (HGF)-induced epithelial cell motility, chemotaxis and wound repair. Upon S.flexneri cell infection, its actin-severing activity enhances actin-based motility of the bacteria and plays a role during the dissemination. [UniProt] |
| Cellular Localization | Cytoplasm, cytoskeleton. Cell projection, lamellipodium, ruffle, microvillus, filopodium tip, filopodium. Note=Relocalized in the tip of cellular protrusions and filipodial extensions upon infection with S.flexneri in primary intestinal epithelial cells (IEC) and in the tail-like structures forming the actin comets of S. flexneri. Redistributed to the leading edge of hepatocyte growth factor (HGF)-induced lamellipodia. [UniProt] |
| Calculated MW | 93 kDa |
| PTM | Tyrosine phosphorylation is induced by epidermal growth factor (EGF) and stimulates cell migration (By similarity). Phosphorylated on tyrosine residues by SRC. The unphosphorylated form increases the initial rate of actin-nucleating activity, whereas the tyrosine-phosphorylated form inhibits actin-nucleating activity, enhances actin-bundling activity and enhances actin-severing activity by reducing high Ca(2+) requirements. The tyrosine-phosphorylated form does not regulate actin-capping activity. Tyrosine phosphorylation is essential for cell migration: tyrosine phosphorylation sites in the N-terminus half regulate actin reorganization and cell morphology, whereas tyrosine phosphorylation sites in the C-terminus half regulate cell migration via interaction with PLCG1. [UniProt] |
Images (1) Click the Picture to Zoom In