ARG52442
anti-Tau phospho (Ser416) antibody
anti-Tau phospho (Ser416) antibody for Western blot and Rat
Neuroscience antibody; Signaling Transduction antibody; Neuron Development Study antibody
Overview
| Product Description | Rabbit Polyclonal antibody recognizes Tau phospho (Ser416) |
|---|---|
| Tested Reactivity | Rat |
| Predict Reactivity | Hu, Ms, Bov, Dog, NHuPrm |
| Tested Application | WB |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Target Name | Tau |
| Antigen Species | Rat |
| Immunogen | Synthetic phospho-peptide corresponding to amino acid residues surrounding Ser416 conjugated to KLH |
| Conjugation | Un-conjugated |
| Alternate Names | TAU; Neurofibrillary tangle protein; Paired helical filament-tau; PPND; DDPAC; FTDP-17; MTBT2; Microtubule-associated protein tau; PHF-tau; MSTD; PPP1R103; MTBT1; MAPTL |
Application Instructions
| Application Suggestion |
|
||||
|---|---|---|---|---|---|
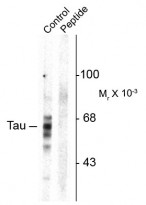
| Application Note | Specific for ~59, 65, 68k tau protein phosphorylated at Ser416. * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
Properties
| Form | Liquid |
|---|---|
| Purification | Affinity Purified |
| Buffer | 10 mM HEPES (pH 7.5), 150 mM NaCl, 0.1 mg/ml BSA and 50% Glycerol |
| Stabilizer | 0.1 mg/ml BSA, 50% Glycerol |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | MAPT |
| Gene Full Name | microtubule-associated protein tau |
| Background | Tau is a key microtubule-associated protein that plays an important role in the formation of microtubules in axons (Binder et al. 1985). Six tau isoforms have been identified as products of a single gene produced by alternative mRNA splicing (Goedert 1990). Tau mutations have been implicated in many neurodegenerative disorders such as Alzheimer’s disease (AD), Pick’s disease and progressive supranuclear palsy. It has been well documented that hyperphosphorylated tau is a major component of paired helical filaments in AD brain (Lee 1995). Serine 416 has been demonstrated to be a major phosphorylation site in vitro by CaM kinase II (Steiner at al. 1990). |
| Research Area | Neuroscience antibody; Signaling Transduction antibody; Neuron Development Study antibody |
| Calculated MW | 79 kDa |
| PTM | Phosphorylation at serine and threonine residues in S-P or T-P motifs by proline-directed protein kinases (PDPK1: CDK1, CDK5, GSK3, MAPK) (only 2-3 sites per protein in interphase, seven-fold increase in mitosis, and in the form associated with paired helical filaments (PHF-tau)), and at serine residues in K-X-G-S motifs by MAP/microtubule affinity-regulating kinase (MARK1 or MARK2), causing detachment from microtubules, and their disassembly. Phosphorylation decreases with age. Phosphorylation within tau/MAP's repeat domain or in flanking regions seems to reduce tau/MAP's interaction with, respectively, microtubules or plasma membrane components. Phosphorylation on Ser-610, Ser-622, Ser-641 and Ser-673 in several isoforms during mitosis. Phosphorylation at Ser-548 by GSK3B reduces ability to bind and stabilize microtubules. Phosphorylation at Ser-579 by BRSK1 and BRSK2 in neurons affects ability to bind microtubules and plays a role in neuron polarization. Phosphorylated at Ser-554, Ser-579, Ser-602, Ser-606 and Ser-669 by PHK. Phosphorylation at Ser-214 by SGK1 mediates microtubule depolymerization and neurite formation in hippocampal neurons. There is a reciprocal down-regulation of phosphorylation and O-GlcNAcylation. Phosphorylation on Ser-717 completely abolishes the O-GlcNAcylation on this site, while phosphorylation on Ser-713 and Ser-721 reduces glycosylation by a factor of 2 and 4 respectively. Phosphorylation on Ser-721 is reduced by about 41.5% by GlcNAcylation on Ser-717. Dephosphorylated at several serine and threonine residues by the serine/threonine phosphatase PPP5C. Polyubiquitinated. Requires functional TRAF6 and may provoke SQSTM1-dependent degradation by the proteasome (By similarity). PHF-tau can be modified by three different forms of polyubiquitination. 'Lys-48'-linked polyubiquitination is the major form, 'Lys-6'-linked and 'Lys-11'-linked polyubiquitination also occur. O-glycosylated. O-GlcNAcylation content is around 8.2%. There is reciprocal down-regulation of phosphorylation and O-GlcNAcylation. Phosphorylation on Ser-717 completely abolishes the O-GlcNAcylation on this site, while phosphorylation on Ser-713 and Ser-721 reduces O-GlcNAcylation by a factor of 2 and 4 respectively. O-GlcNAcylation on Ser-717 decreases the phosphorylation on Ser-721 by about 41.5%. Glycation of PHF-tau, but not normal brain TAU/MAPT. Glycation is a non-enzymatic post-translational modification that involves a covalent linkage between a sugar and an amino group of a protein molecule forming ketoamine. Subsequent oxidation, fragmentation and/or cross-linking of ketoamine leads to the production of advanced glycation endproducts (AGES). Glycation may play a role in stabilizing PHF aggregation leading to tangle formation in AD. |
Images (1) Click the Picture to Zoom In
-
ARG52442 anti-Tau phospho (Ser416) antibody WB image
Western blot: Rat brain homogenate showing specific immunolabeling of the ~59, 65, 68 kDa Tau isoforms phosphorylated at Ser416 (control) stained with ARG52442 anti-Tau phospho (Ser416) antibody. Imunolabeling is blocked by preadsorption with the phospho-peptide used as antigen (Peptide) but not by the corresponding dephospho-peptide (not shown).