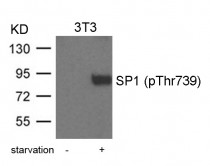
ARG51729
anti-SP1 phospho (Thr739) antibody
anti-SP1 phospho (Thr739) antibody for Western blot and Human,Rat
Developmental Biology antibody; Gene Regulation antibody; Metabolism antibody; Signaling Transduction antibody
Overview
| Product Description | Rabbit Polyclonal antibody recognizes SP1 phospho (Thr739) |
|---|---|
| Tested Reactivity | Hu, Rat |
| Tested Application | WB |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Target Name | SP1 |
| Antigen Species | Human |
| Immunogen | Peptide sequence around phosphorylation site of threonine 739 (T-A-T(p)-P-S) derived from human SP1 |
| Conjugation | Un-conjugated |
| Alternate Names | Transcription factor Sp1 |
Application Instructions
| Application Suggestion |
|
||||
|---|---|---|---|---|---|
| Application Note | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
Properties
| Form | Liquid |
|---|---|
| Purification | Antibodies were produced by immunizing rabbits with KLH-conjugated synthetic phosphopeptide. Antibodies were purified by affinity-chromatography using epitope-specific phosphopeptide. In addition, non-phospho specific antibodies were removed by chromatogramphy using non-phosphopeptide. |
| Buffer | PBS (without Mg2+ and Ca2+, pH 7.4), 150mM NaCl, 0.02% Sodium azide and 50% Glycerol. |
| Preservative | 0.02% Sodium azide |
| Stabilizer | 50% Glycerol |
| Concentration | 1 mg/ml |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | SP1 |
| Gene Full Name | Sp1 transcription factor |
| Background | Transcription factor that can activate or repress transcription in response to physiological and pathological stimuli. Binds with high affinity to GC-rich motifs and regulates the expression of a large number of genes involved in a variety of processes such as cell growth, apoptosis, differentiation and immune responses. Highly regulated by post-translational modifications (phosphorylations, sumoylation, proteolytic cleavage, glycosylation and acetylation). Binds also the PDGFR-alpha G-box promoter. May have a role in modulating the cellular response to DNA damage. Implicated in chromatin remodeling. Plays a role in the recruitment of SMARCA4/BRG1 on the c-FOS promoter. Plays an essential role in the regulation of FE65 gene expression. |
| Function | Transcription factor that can activate or repress transcription in response to physiological and pathological stimuli. Binds with high affinity to GC-rich motifs and regulates the expression of a large number of genes involved in a variety of processes such as cell growth, apoptosis, differentiation and immune responses. Highly regulated by post-translational modifications (phosphorylations, sumoylation, proteolytic cleavage, glycosylation and acetylation). Binds also the PDGFR-alpha G-box promoter. May have a role in modulating the cellular response to DNA damage. Implicated in chromatin remodeling. Plays a role in the recruitment of SMARCA4/BRG1 on the c-FOS promoter. Plays an essential role in the regulation of FE65 gene expression. In complex with ATF7IP, maintains telomerase activity in cancer cells by inducing TERT and TERC gene expression. Isoform 3 is a stronger activator of transcription than isoform 1. Positively regulates the transcription of the core clock component ARNTL/BMAL1. [UniProt] |
| Research Area | Developmental Biology antibody; Gene Regulation antibody; Metabolism antibody; Signaling Transduction antibody |
| Calculated MW | 81 kDa |
| PTM | Phosphorylated on multiple serine and threonine residues. Phosphorylation is coupled to ubiquitination, sumoylation and proteolytic processing. Phosphorylation on Ser-59 enhances proteolytic cleavage. Phosphorylation on Ser-7 enhances ubiquitination and protein degradation. Hyperphosphorylation on Ser-101 in response to DNA damage has no effect on transcriptional activity. MAPK1/MAPK3-mediated phosphorylation on Thr-453 and Thr-739 enhances VEGF transcription but, represses FGF2-triggered PDGFR-alpha transcription. Also implicated in the repression of RECK by ERBB2. Hyperphosphorylated on Thr-278 and Thr-739 during mitosis by MAPK8 shielding SP1 from degradation by the ubiquitin-dependent pathway. Phosphorylated in the zinc-finger domain by calmodulin-activated PKCzeta. Phosphorylation on Ser-641 by PKCzeta is critical for TSA-activated LHR gene expression through release of its repressor, p107. Phosphorylation on Thr-668, Ser-670 and Thr-681 is stimulated by angiotensin II via the AT1 receptor inducing increased binding to the PDGF-D promoter. This phosphorylation is increased in injured artey wall. Ser-59 and Thr-681 can both be dephosphorylated by PP2A during cell-cycle interphase. Dephosphorylation on Ser-59 leads to increased chromatin association during interphase and increases the transcriptional activity. On insulin stimulation, sequentially glycosylated and phosphorylated on several C-terminal serine and threonine residues. Acetylated. Acetylation/deacetylation events affect transcriptional activity. Deacetylation leads to an increase in the expression the 12(s)-lipooxygenase gene though recruitment of p300 to the promoter. Ubiquitinated. Ubiquitination occurs on the C-terminal proteolytically-cleaved peptide and is triggered by phosphorylation. Sumoylated with SUMO1. Sumoylation modulates proteolytic cleavage of the N-terminal repressor domain. Sumoylation levels are attenuated during tumorigenesis. Phosphorylation mediates SP1 desumoylation. Proteolytic cleavage in the N-terminal repressor domain is prevented by sumoylation. The C-terminal cleaved product is susceptible to degradation. O-glycosylated; Contains 8 N-acetylglucosamine side chains. Levels are controlled by insulin and the SP1 phosphorylation states. Insulin-mediated O-glycosylation locates SP1 to the nucleus, where it is sequentially deglycosylated and phosphorylated. O-glycosylation affects transcriptional activity through disrupting the interaction with a number of transcription factors including ELF1 and NFYA. Also inhibits interaction with the HIV1 promoter. Inhibited by peroxisomome proliferator receptor gamma (PPARgamma). |
Images (1) Click the Picture to Zoom In