ARG52402
anti-Progesterone Receptor phospho (Ser294) antibody [608]
anti-Progesterone Receptor phospho (Ser294) antibody [608] for IHC-Formalin-fixed paraffin-embedded sections,Western blot and Human
Cancer antibody; Gene Regulation antibody; Neuroscience antibody; Signaling Transduction antibody
Overview
| Product Description | Mouse Monoclonal antibody [608] recognizes Progesterone Receptor phospho (Ser294) |
|---|---|
| Tested Reactivity | Hu |
| Predict Reactivity | NHuPrm |
| Tested Application | IHC-P, WB |
| Host | Mouse |
| Clonality | Monoclonal |
| Clone | 608 |
| Isotype | IgG1 |
| Target Name | Progesterone Receptor |
| Antigen Species | Human |
| Immunogen | Synthetic phospho-peptide corresponding to amino acid residues surrounding Ser294 conjugated to KLH |
| Conjugation | Un-conjugated |
| Alternate Names | PR; NR3C3; Nuclear receptor subfamily 3 group C member 3; Progesterone receptor |
Application Instructions
| Application Suggestion |
|
||||||
|---|---|---|---|---|---|---|---|
| Application Note | Specific for the ~90k PR-A isoform and the ~120k PR-B isoform phosphorylated at Ser294. Immunolabeling is blocked by the phosphopeptide used as the antigen but not by the corresponding dephosphopeptide. * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
Properties
| Form | Liquid |
|---|---|
| Purification | Protein G purified |
| Buffer | 10 mM HEPES (pH 7.5), 150 mM NaCl, 0.1 mg/ml BSA and 50% Glycerol |
| Stabilizer | 0.1 mg/ml BSA, 50% Glycerol |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | PGR |
| Gene Full Name | progesterone receptor |
| Background | There is accumulating evidence to suggest that progesterone plays an essential role in the regulation of growth and differentiation of mammary glands and thus may play a key role in breast cancer (Edwards, 2005). The biological response to progesterone is mediated by two distinct forms of the human progesterone receptor (PR-A and PR-B forms). In most cell contexts, the B form functions as a transcriptional activator, whereas the A form functions as a transcriptional inhibitor of steroid hormones (Attia et al., 2000; Lin et al., 2003). Recently it has been demonstrated that there is differential hormone dependent regulation of the phosphorylation of the A and B forms of the receptor (Clemm et al., 2000) . Treatment of T47D breast cancer cells with progestin agonist increases the phosphorylation of Ser190 and Ser294 with different kinetics. These phosphorylation events may differentially affect the transcriptional activity of the receptor. |
| Research Area | Cancer antibody; Gene Regulation antibody; Neuroscience antibody; Signaling Transduction antibody |
| Calculated MW | 99 kDa |
| PTM | Phosphorylated on multiple serine sites. Several of these sites are hormone-dependent. Phosphorylation on Ser-294 occurs preferentially on isoform B, is highly hormone-dependent and modulates ubiquitination and sumoylation on Lys-388. Phosphorylation on Ser-102 and Ser-345 also requires induction by hormone. Basal phosphorylation on Ser-81, Ser-162, Ser-190 and Ser-400 is increased in response to progesterone and can be phosphorylated in vitro by the CDK2-A1 complex. Increased levels of phosphorylation on Ser-400 also in the presence of EGF, heregulin, IGF, PMA and FBS. Phosphorylation at this site by CDK2 is ligand-independent, and increases nuclear translocation and transcriptional activity. Phosphorylation at Ser-162 and Ser-294, but not at Ser-190, is impaired during the G(2)/M phase of the cell cycle. Phosphorylation on Ser-345 by ERK1/2 MAPK is required for interaction with SP1. Sumoylation is hormone-dependent and represses transcriptional activity. Sumoylation on all three sites is enhanced by PIAS3. Desumoylated by SENP1. Sumoylation on Lys-388, the main site of sumoylation, is repressed by ubiquitination on the same site, and modulated by phosphorylation at Ser-294. Ubiquitination is hormone-dependent and represses sumoylation on the same site. Promoted by MAPK-mediated phosphorylation on Ser-294. Palmitoylated by ZDHHC7 and ZDHHC21. Palmitoylation is required for plasma membrane targeting and for rapid intracellular signaling via ERK and AKT kinases and cAMP generation. |
Images (1) Click the Picture to Zoom In
-
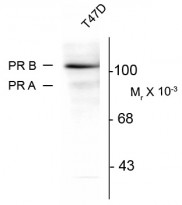
ARG52402 anti-Progesterone Receptor phospho (Ser294) antibody [608] WB image
Western blot: whole cell T47D lysate prepared from cells that had been incubated in the presence of the synthetic progestin agonist R5020 (500 nM) showing specific immunolabeling of the ~90k PR-A isoform and the ~120 PR-B isoform of the progesterone receptor phosphorylated at Ser294 stained with ARG52402 anti-Progesterone Receptor phospho (Ser294) antibody [608]. The immunolabeling is blocked by the phosphopeptide used as the antigen (not shown).
Clone References