ARG58268
anti-NFkB p65 phospho (Ser276) antibody
anti-NFkB p65 phospho (Ser276) antibody for ICC/IF,IHC-Formalin-fixed paraffin-embedded sections,Western blot and Human,Rat
Cancer antibody; Cell Biology and Cellular Response antibody; Cell Death antibody; Gene Regulation antibody; Immune System antibody; Metabolism antibody; Microbiology and Infectious Disease antibody; Neuroscience antibody; Signaling Transduction antibody; NFkB nuclear translocation Study antibody; Inflammation Study antibody
Overview
| Product Description | Rabbit Polyclonal antibody recognizes NFkB p65 phospho (Ser276) |
|---|---|
| Tested Reactivity | Hu, Rat |
| Tested Application | ICC/IF, IHC-P, WB |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Target Name | NFkB p65 |
| Antigen Species | Human |
| Immunogen | Phospho specific peptide peptide around Ser276 of Human NFkB p65 (NP_068810.3). |
| Conjugation | Un-conjugated |
| Alternate Names | Nuclear factor NF-kappa-B p65 subunit; Nuclear factor of kappa light polypeptide gene enhancer in B-cells 3; NFKB3; p65; Transcription factor p65 |
Application Instructions
| Application Suggestion |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Application Note | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. | ||||||||
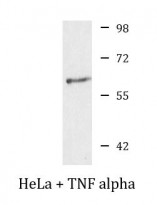
| Positive Control | HeLa + TNF alpha | ||||||||
| Observed Size | 60 kDa |
Properties
| Form | Liquid |
|---|---|
| Purification | Affinity purified. |
| Buffer | PBS (pH 7.3), 0.02% Sodium azide and 50% Glycerol. |
| Preservative | 0.02% Sodium azide |
| Stabilizer | 50% Glycerol |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | RELA |
| Gene Full Name | v-rel avian reticuloendotheliosis viral oncogene homolog A |
| Background | NFkB is a ubiquitous transcription factor involved in several biological processes. It is held in the cytoplasm in an inactive state by specific inhibitors. Upon degradation of the inhibitor, NF-kappa-B moves to the nucleus and activates transcription of specific genes. NF-kappa-B is composed of NFKB1 or NFKB2 bound to either REL, RELA, or RELB. The most abundant form of NF-kappa-B is NFKB1 complexed with the product of this gene, RELA. Four transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Sep 2011] |
| Function | NFkB is a pleiotropic transcription factor present in almost all cell types and is the endpoint of a series of signal transduction events that are initiated by a vast array of stimuli related to many biological processes such as inflammation, immunity, differentiation, cell growth, tumorigenesis and apoptosis. NF-kappa-B is a homo- or heterodimeric complex formed by the Rel-like domain-containing proteins RELA/p65, RELB, NFKB1/p105, NFKB1/p50, REL and NFKB2/p52. The heterodimeric RELA-NFKB1 complex appears to be most abundant one. The dimers bind at kappa-B sites in the DNA of their target genes and the individual dimers have distinct preferences for different kappa-B sites that they can bind with distinguishable affinity and specificity. Different dimer combinations act as transcriptional activators or repressors, respectively. The NF-kappa-B heterodimeric RELA-NFKB1 and RELA-REL complexes, for instance, function as transcriptional activators. NF-kappa-B is controlled by various mechanisms of post-translational modification and subcellular compartmentalization as well as by interactions with other cofactors or corepressors. NF-kappa-B complexes are held in the cytoplasm in an inactive state complexed with members of the NF-kappa-B inhibitor (I-kappa-B) family. In a conventional activation pathway, I-kappa-B is phosphorylated by I-kappa-B kinases (IKKs) in response to different activators, subsequently degraded thus liberating the active NF-kappa-B complex which translocates to the nucleus. The inhibitory effect of I-kappa-B on NF-kappa-B through retention in the cytoplasm is exerted primarily through the interaction with RELA. RELA shows a weak DNA-binding site which could contribute directly to DNA binding in the NF-kappa-B complex. Beside its activity as a direct transcriptional activator, it is also able to modulate promoters accessibility to transcription factors and thereby indirectly regulate gene expression. Associates with chromatin at the NF-kappa-B promoter region via association with DDX1. Essential for cytokine gene expression in T-cells (PubMed:15790681). The NF-kappa-B homodimeric RELA-RELA complex appears to be involved in invasin-mediated activation of IL-8 expression. [UniProt] |
| Cellular Localization | Cytoplasm, Nucleus. [UniProt] |
| Highlight | Related products: NFkB p65 antibodies; NFkB p65 Duos / Panels; Anti-Rabbit IgG secondary antibodies; Related news: Exploring Antiviral Immune Response |
| Research Area | Cancer antibody; Cell Biology and Cellular Response antibody; Cell Death antibody; Gene Regulation antibody; Immune System antibody; Metabolism antibody; Microbiology and Infectious Disease antibody; Neuroscience antibody; Signaling Transduction antibody; NFkB nuclear translocation Study antibody; Inflammation Study antibody |
| Calculated MW | 60 kDa |
| PTM | Ubiquitinated, leading to its proteasomal degradation. Degradation is required for termination of NF-kappa-B response. Monomethylated at Lys-310 by SETD6. Monomethylation at Lys-310 is recognized by the ANK repeats of EHMT1 and promotes the formation of repressed chromatin at target genes, leading to down-regulation of NF-kappa-B transcription factor activity. Phosphorylation at Ser-311 disrupts the interaction with EHMT1 without preventing monomethylation at Lys-310 and relieves the repression of target genes (By similarity). Phosphorylation at Ser-311 disrupts the interaction with EHMT1 and promotes transcription factor activity (By similarity). Phosphorylation on Ser-536 stimulates acetylation on Lys-310 and interaction with CBP; the phosphorylated and acetylated forms show enhanced transcriptional activity. Phosphorylation at Ser-276 by RPS6KA4 and RPS6KA5 promotes its transactivation and transcriptional activities. Reversibly acetylated; the acetylation seems to be mediated by CBP, the deacetylation by HDAC3 and SIRT2. Acetylation at Lys-122 enhances DNA binding and impairs association with NFKBIA. Acetylation at Lys-310 is required for full transcriptional activity in the absence of effects on DNA binding and NFKBIA association. Acetylation at Lys-310 promotes interaction with BRD4. Acetylation can also lower DNA-binding and results in nuclear export. Interaction with BRMS1 promotes deacetylation of Lys-310. Lys-310 is deacetylated by SIRT2. S-nitrosylation of Cys-38 inactivates the enzyme activity. Sulfhydration at Cys-38 mediates the anti-apoptotic activity by promoting the interaction with RPS3 and activating the transcription factor activity. Sumoylation by PIAS3 negatively regulates DNA-bound activated NF-kappa-B. Proteolytically cleaved within a conserved N-terminus region required for base-specific contact with DNA in a CPEN1-mediated manner, and hence inhibits NF-kappa-B transcriptional activity (PubMed:18212740). [UniProt] |
Images (3) Click the Picture to Zoom In
-
ARG58268 anti-NFkB p65 phospho (Ser276) antibody WB image
Western blot: 25 µg of HeLa cells were treated by TNF alpha (20 ng/ml) for 30 min. The blot was stained with ARG58268 anti-NFkB p65 phospho (Ser276) antibody.
-
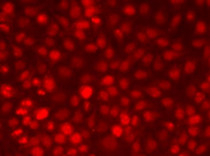
ARG58268 anti-NFkB p65 phospho (Ser276) antibody ICC/IF image
Immunofluorescence: U2OS cells stained with ARG58268 anti-NFkB p65 phospho (Ser276) antibody at 1:100 dilution.
-
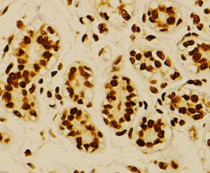
ARG58268 anti-NFkB p65 phospho (Ser276) antibody IHC-P image
Immunohistochemistry: Paraffin-embedded Human breast cancer stained with ARG58268 anti-NFkB p65 phospho (Ser276) antibody at 1:100 dilution.