ARG55717
anti-Insulin Receptor antibody
anti-Insulin Receptor antibody for Western blot and Human
Overview
| Product Description | Rabbit Polyclonal antibody recognizes Insulin Receptor |
|---|---|
| Tested Reactivity | Hu |
| Predict Reactivity | Ms, Rat |
| Tested Application | WB |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Target Name | Insulin Receptor |
| Antigen Species | Human |
| Immunogen | KLH-conjugated synthetic peptide corresponding to aa. 826-854 (N-terminus) of Human Insulin Receptor. |
| Conjugation | Un-conjugated |
| Alternate Names | Insulin receptor; IR; CD220; HHF5; CD antigen CD220; EC 2.7.10.1 |
Application Instructions
| Application Suggestion |
|
||||
|---|---|---|---|---|---|
| Application Note | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. | ||||
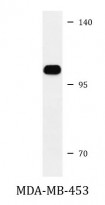
| Positive Control | MDA-MB-453 |
Properties
| Form | Liquid |
|---|---|
| Purification | Purification with Protein A and immunogen peptide. |
| Buffer | PBS and 0.09% (W/V) Sodium azide. |
| Preservative | 0.09% (W/V) Sodium azide. |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | INSR |
| Gene Full Name | insulin receptor |
| Background | After removal of the precursor signal peptide, the insulin receptor precursor is post-translationally cleaved into two chains (alpha and beta) that are covalently linked. Binding of insulin to the insulin receptor (INSR) stimulates glucose uptake. Two transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Jul 2008] |
| Function | Receptor tyrosine kinase which mediates the pleiotropic actions of insulin. Binding of insulin leads to phosphorylation of several intracellular substrates, including, insulin receptor substrates (IRS1, 2, 3, 4), SHC, GAB1, CBL and other signaling intermediates. Each of these phosphorylated proteins serve as docking proteins for other signaling proteins that contain Src-homology-2 domains (SH2 domain) that specifically recognize different phosphotyrosines residues, including the p85 regulatory subunit of PI3K and SHP2. Phosphorylation of IRSs proteins lead to the activation of two main signaling pathways: the PI3K-AKT/PKB pathway, which is responsible for most of the metabolic actions of insulin, and the Ras-MAPK pathway, which regulates expression of some genes and cooperates with the PI3K pathway to control cell growth and differentiation. Binding of the SH2 domains of PI3K to phosphotyrosines on IRS1 leads to the activation of PI3K and the generation of phosphatidylinositol-(3, 4, 5)-triphosphate (PIP3), a lipid second messenger, which activates several PIP3-dependent serine/threonine kinases, such as PDPK1 and subsequently AKT/PKB. The net effect of this pathway is to produce a translocation of the glucose transporter SLC2A4/GLUT4 from cytoplasmic vesicles to the cell membrane to facilitate glucose transport. Moreover, upon insulin stimulation, activated AKT/PKB is responsible for: anti-apoptotic effect of insulin by inducing phosphorylation of BAD; regulates the expression of gluconeogenic and lipogenic enzymes by controlling the activity of the winged helix or forkhead (FOX) class of transcription factors. Another pathway regulated by PI3K-AKT/PKB activation is mTORC1 signaling pathway which regulates cell growth and metabolism and integrates signals from insulin. AKT mediates insulin-stimulated protein synthesis by phosphorylating TSC2 thereby activating mTORC1 pathway. The Ras/RAF/MAP2K/MAPK pathway is mainly involved in mediating cell growth, survival and cellular differentiation of insulin. Phosphorylated IRS1 recruits GRB2/SOS complex, which triggers the activation of the Ras/RAF/MAP2K/MAPK pathway. In addition to binding insulin, the insulin receptor can bind insulin-like growth factors (IGFI and IGFII). Isoform Short has a higher affinity for IGFII binding. When present in a hybrid receptor with IGF1R, binds IGF1. Hybrid receptors composed of IGF1R and INSR isoform Long are activated with a high affinity by IGF1, with low affinity by IGF2 and not significantly activated by insulin, and that hybrid receptors composed of IGF1R and INSR isoform Short are activated by IGF1, IGF2 and insulin. In contrast, one research shows that hybrid receptors composed of IGF1R and INSR isoform Long and hybrid receptors composed of IGF1R and INSR isoform Short have similar binding characteristics, both bind IGF1 and have a low affinity for insulin. [UniProt] |
| Cellular Localization | Cell membrane; Single-pass type I membrane protein |
| Calculated MW | 156 kDa |
| PTM | After being transported from the endoplasmic reticulum to the Golgi apparatus, the single glycosylated precursor is further glycosylated and then cleaved, followed by its transport to the plasma membrane. Autophosphorylated on tyrosine residues in response to insulin. Phosphorylation of Tyr-999 is required for binding to IRS1, SHC1 and STAT5B. Dephosphorylated by PTPRE at Tyr-999, Tyr-1185, Tyr-1189 and Tyr-1190. Dephosphorylated by PTPRF and PTPN1. Dephosphorylated by PTPN2; down-regulates insulin-induced signaling. |
Images (1) Click the Picture to Zoom In