ARG22258
anti-HSF1 antibody [10H8]
anti-HSF1 antibody [10H8] for ELISA,Gel supershift assays,ICC/IF,Immunoprecipitation,Western blot and Human,Mouse,Rat,Bovine,Guinea pig,Hamster,Monkey,Rabbit
Overview
| Product Description | Rat Monoclonal antibody [10H8] recognizes HSF1 |
|---|---|
| Tested Reactivity | Hu, Ms, Rat, Bov, Gpig, Hm, Mk, Rb |
| Tested Application | ELISA, GSA, ICC/IF, IP, WB |
| Specificity | Detects ~85kDa (unstressed cell lysates), and~95kDa (heat shocked cell lysates). |
| Host | Rat |
| Clonality | Monoclonal |
| Clone | 10H8 |
| Isotype | IgG1 |
| Target Name | HSF1 |
| Antigen Species | Mouse |
| Immunogen | Purified recombinant Mouse HSF1 protein |
| Epitope | around aa. 378-395 |
| Conjugation | Un-conjugated |
| Alternate Names | Heat shock transcription factor 1; Heat shock factor protein 1; HSF 1; HSTF 1; HSTF1 |
Application Instructions
| Application Suggestion |
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Application Note | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
Properties
| Form | Liquid |
|---|---|
| Purification | Purification with Protein G. |
| Buffer | PBS (pH 7.4), 0.09% Sodium azide and 50% Glycerol |
| Preservative | 0.09% Sodium azide |
| Stabilizer | 50% Glycerol |
| Concentration | 1 mg/ml |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | Hsf1 |
| Gene Full Name | heat shock factor 1 |
| Background | The product of this gene is a heat-shock transcription factor. Transcription of heat-shock genes is rapidly induced after temperature stress. Hsp90, by itself and/or associated with multichaperone complexes, is a major repressor of this gene. [provided by RefSeq, Jul 2008] |
| Function | DNA-binding protein that specifically binds heat shock promoter elements (HSE) and activates transcription. In higher eukaryotes, HSF is unable to bind to the HSE unless the cells are heat shocked. [UniProt] |
| Cellular Localization | Cytoplasm, Nucleus |
| Calculated MW | ~85 kDa (unstressed cell lysates), and ~95 kDa (heat shocked cell lysates). |
| PTM | Phosphorylated (PubMed:9499401, PubMed:10359787, PubMed:11583998, PubMed:26159920). Phosphorylated in unstressed cells; this phosphorylation is constitutive and implicated in the repression of HSF1 transcriptional activity (PubMed:8946918, PubMed:8940068, PubMed:9121459, PubMed:16278218). Phosphorylated on Ser-121 by MAPKAPK2; this phosphorylation promotes interaction with HSP90 proteins and inhibits HSF1 homotrimerization, DNA-binding and transactivation activities (PubMed:16278218). Phosphorylation on Ser-303 by GSK3B/GSK3-beta and on Ser-307 by MAPK3 within the regulatory domain is involved in the repression of HSF1 transcriptional activity and occurs in a RAF1-dependent manner (PubMed:8946918, PubMed:8940068, PubMed:9121459, PubMed:9535852, PubMed:10747973, PubMed:12646186). Phosphorylation on Ser-303 and Ser-307 increases HSF1 nuclear export in a YWHAE- and XPO1/CRM1-dependent manner (PubMed:12917326). Phosphorylation on Ser-307 is a prerequisite for phosphorylation on Ser-303 (PubMed:8940068). According to PubMed:9535852, Ser-303 is not phosphorylated in unstressed cells. Phosphorylated on Ser-419 by PLK1; phosphorylation promotes nuclear translocation upon heat shock (PubMed:15661742). Hyperphosphorylated upon heat shock and during the attenuation and recovery phase period of the heat shock response (PubMed:11447121, PubMed:12659875, PubMed:24581496). Phosphorylated on Thr-142; this phosphorylation increases HSF1 transactivation activity upon heat shock (PubMed:12659875). Phosphorylation on Ser-230 by CAMK2A; this phosphorylation enhances HSF1 transactivation activity upon heat shock (PubMed:11447121). Phosphorylation on Ser-326 by MAPK12; this phosphorylation enhances HSF1 nuclear translocation, homotrimerization and transactivation activities upon heat shock (PubMed:15760475, PubMed:27354066). Phosphorylated on Ser-320 by PRKACA/PKA; this phosphorylation promotes nuclear localization and transcriptional activity upon heat shock (PubMed:21085490). Phosphorylated on Ser-363 by MAPK8; this phosphorylation occurs upon heat shock, induces HSF1 translocation into nuclear stress bodies and negatively regulates transactivation activity (PubMed:10747973). Neither basal nor stress-inducible phosphorylation on Ser-230, Ser-292, Ser-303, Ser-307, Ser-314, Ser-319, Ser-320, Thr-323, Ser-326, Ser-338, Ser-344, Ser-363, Thr-367, Ser-368 and Thr-369 within the regulatory domain is involved in the regulation of HSF1 subcellular localization or DNA-binding activity; however, it negatively regulates HSF1 transactivation activity (PubMed:25963659). Phosphorylated on Ser-216 by PLK1 in the early mitotic period; this phosphorylation regulates HSF1 localization to the spindle pole, the recruitment of the SCF(BTRC) ubiquitin ligase complex inducing HSF1 degradation, and hence mitotic progression (PubMed:18794143). Dephosphorylated on Ser-121, Ser-307, Ser-314, Thr-323 and Thr-367 by phosphatase PPP2CA in an IER5-dependent manner, leading to HSF1-mediated transactivation activity (PubMed:26754925). Sumoylated with SUMO1 and SUMO2 upon heat shock in a ERK2-dependent manner (PubMed:12646186, PubMed:12665592). Sumoylated by SUMO1 on Lys-298; sumoylation occurs upon heat shock and promotes its localization to nuclear stress bodies and DNA-binding activity (PubMed:11514557). Phosphorylation on Ser-303 and Ser-307 is probably a prerequisite for sumoylation (PubMed:12646186, PubMed:12665592). Acetylated on Lys-118; this acetylation is decreased in a IER5-dependent manner (PubMed:26754925). Acetylated on Lys-118, Lys-208 and Lys-298; these acetylations occur in a EP300-dependent manner (PubMed:24581496, PubMed:27189267). Acetylated on Lys-80; this acetylation inhibits DNA-binding activity upon heat shock (PubMed:19229036). Deacetylated on Lys-80 by SIRT1; this deacetylation increases DNA-binding activity (PubMed:19229036). Ubiquitinated by SCF(BTRC) and degraded following stimulus-dependent phosphorylation at Ser-216 by PLK1 in mitosis (PubMed:18794143). Polyubiquitinated (PubMed:24581496). Undergoes proteasomal degradation upon heat shock and during the attenuation and recovery phase period of the heat shock response (PubMed:24581496). |
Images (3) Click the Picture to Zoom In
-
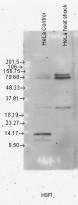
ARG22258 anti-HSF1 antibody [10H8] WB image
Western blot: Human Heat Shocked HeLa cell lysates stained with ARG22258 anti-HSF1 antibody [10H8].
-
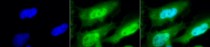
ARG22258 anti-HSF1 antibody [10H8] ICC/IF image
Immunocytochemistry: 2% Formaldehyde (20 min at RT) fixed Heat Shocked HeLa cells stained with ARG22258 anti-HSF1 antibody [10H8] (green) at 1:100 dilution (12 hours at 4°C). Counterstain: DAPI (blue) nuclear stain at 1:40000 for 120 min at RT. Magnification: 100x. Left: DAPI (blue) nuclear stain, Middle: Primary antibody, Right: Composite.
-
ARG22258 anti-HSF1 antibody [10H8] ICC/IF image
Immunocytochemistry: 2% Formaldehyde (20 min at RT) fixed Heat Shocked HeLa cells stained with ARG22258 anti-HSF1 antibody [10H8] (red) at 1:100 dilution (12 hours at 4°C). Counterstain: DAPI (blue) nuclear stain at 1:40000 for 120 min at RT. Magnification: 20x. Left: DAPI (blue) nuclear stain, Middle: Primary antibody, Right: Composite.