ARG59699
anti-CEBP beta antibody
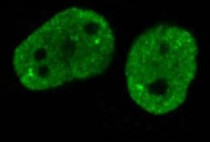
anti-CEBP beta antibody for Flow cytometry,ICC/IF,Western blot and Human
Overview
| Product Description | Rabbit Polyclonal antibody recognizes CEBP beta |
|---|---|
| Tested Reactivity | Hu |
| Tested Application | FACS, ICC/IF, WB |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Target Name | CEBP Beta |
| Antigen Species | Human |
| Immunogen | Synthetic peptide derived from Human CEBP beta. |
| Conjugation | Un-conjugated |
| Alternate Names | TCF5; TCF-5; C/EBP-beta; CCAAT/enhancer-binding protein beta; IL6DBP; Liver-enriched inhibitory protein; C/EBP beta; Liver activator protein; LIP; Transcription factor 5; Nuclear factor NF-IL6; LAP; NF-IL6 |
Application Instructions
| Application Suggestion |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Application Note | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. | ||||||||
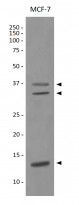
| Observed Size | 37 kDa; 33 kDa; 13 kDa |
Properties
| Form | Liquid |
|---|---|
| Purification | Affinity purified. |
| Buffer | PBS (pH 7.4), 0.02% Sodium azide and 50% Glycerol. |
| Preservative | 0.02% Sodium azide |
| Stabilizer | 50% Glycerol |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links |
Swiss-port # P17676 Human CCAAT/enhancer-binding protein beta |
|---|---|
| Gene Symbol | CEBPB |
| Gene Full Name | CCAAT/enhancer binding protein (C/EBP), beta |
| Background | This intronless gene encodes a transcription factor that contains a basic leucine zipper (bZIP) domain. The encoded protein functions as a homodimer but can also form heterodimers with CCAAT/enhancer-binding proteins alpha, delta, and gamma. Activity of this protein is important in the regulation of genes involved in immune and inflammatory responses, among other processes. The use of alternative in-frame AUG start codons results in multiple protein isoforms, each with distinct biological functions. [provided by RefSeq, Oct 2013] |
| Function | Important transcription factor regulating the expression of genes involved in immune and inflammatory responses. Plays also a significant role in adipogenesis, as well as in the gluconeogenic pathway, liver regeneration, and hematopoiesis. The consensus recognition site is 5'-T[TG]NNGNAA[TG]-3'. Its functional capacity is governed by protein interactions and post-translational protein modifications. During early embryogenesis, plays essential and redundant functions with CEBPA. Has a promitotic effect on many cell types such as hepatocytes and adipocytes but has an antiproliferative effect on T-cells by repressing MYC expression, facilitating differentiation along the T-helper 2 lineage. Binds to regulatory regions of several acute-phase and cytokines genes and plays a role in the regulation of acute-phase reaction and inflammation. Plays also a role in intracellular bacteria killing (By similarity). During adipogenesis, is rapidly expressed and, after activation by phosphorylation, induces CEBPA and PPARG, which turn on the series of adipocyte genes that give rise to the adipocyte phenotype. The delayed transactivation of the CEBPA and PPARG genes by CEBPB appears necessary to allow mitotic clonal expansion and thereby progression of terminal differentiation. Essential for female reproduction because of a critical role in ovarian follicle development (By similarity). Restricts osteoclastogenesis (By similarity). Isoform 2: Essential for gene expression induction in activated macrophages. Plays a major role in immune responses such as CD4(+) T-cell response, granuloma formation and endotoxin shock. Not essential for intracellular bacteria killing. Isoform 3: Acts as a dominant negative through heterodimerization with isoform 2. Promotes osteoblast differentiation and osteoclastogenesis (By similarity). [UniProt] |
| Cellular Localization | Nucleus. Cytoplasm. Note=Translocates to the nucleus when phosphorylated at Ser-288. In T-cells when sumoylated drawn to pericentric heterochromatin thereby allowing proliferation (By similarity). [UniProt] |
| Calculated MW | 36 kDa; 33 kDa; 16 kDa |
| PTM | Methylated. Methylation at Arg-3 by CARM1 and at Lys-43 by EHMT2 inhibit transactivation activity. Methylation is probably inhibited by phosphorylation at Thr-235. Sumoylated by polymeric chains of SUMO2 or SUMO3 (PubMed:12810706). Sumoylation at Lys-174 is required for inhibition of T-cells proliferation. In adipocytes, sumoylation at Lys-174 by PIAS1 leads to ubiquitination and subsequent proteasomal degradation. Desumoylated by SENP2, which abolishes ubiquitination and stabilizes protein levels (By similarity). Ubiquitinated, leading to proteasomal degradation. Phosphorylated at Thr-235 by MAPK and CDK2, serves to prime phosphorylation at Thr-226 and Ser-231 by GSK3B and acquire DNA-binding as well as transactivation activities, required to induce adipogenesis. MAPK and CDK2 act sequentially to maintain Thr-235 in the primed phosphorylated state during mitotical cloning expansion and thereby progression of terminal differentiation. Phosphorylation at Thr-266 enhances transactivation activity. Phosphorylation at Ser-325 in response to calcium increases transactivation activity. Phosphorylated at Thr-235 by RPS6KA1 (PubMed:11684016). O-glycosylated, glycosylation at Ser-227 and Ser-228 prevents phosphorylation on Thr-235, Ser-231 and Thr-226 and DNA binding activity which delays the adipocyte differentiation program. Acetylated. Acetylation at Lys-43 is an important and dynamic regulatory event that contributes to its ability to transactivate target genes, including those associated with adipogenesis and adipocyte function. Deacetylation by HDAC1 represses its transactivation activity. Acetylated by KAT2A and KAT2B within a cluster of lysine residues between amino acids 129-133, this acetylation is strongly induced by glucocorticoid treatment and enhances transactivation activity. [UniProt] |
Images (2) Click the Picture to Zoom In