ARG22986
anti-CD284 / TLR4 antibody [HTA125]
anti-CD284 / TLR4 antibody [HTA125] for CyTOF®-candidate,Flow cytometry,Immunoprecipitation,Western blot and Human,Dog,Guinea pig,Pig,Rhesus Monkey
Overview
| Product Description | Mouse Monoclonal antibody [HTA125] recognizes CD284 / TLR4 |
|---|---|
| Tested Reactivity | Hu, Dog, Gpig, Pig, R. Mk |
| Tested Application | CyTOF®-candidate, FACS, IP, WB |
| Host | Mouse |
| Clonality | Monoclonal |
| Clone | HTA125 |
| Isotype | IgG2a |
| Target Name | CD284 / TLR4 |
| Antigen Species | Human |
| Immunogen | Ba/F3 cell line expressing TLR4 (CD284). |
| Conjugation | Un-conjugated |
| Alternate Names | CD284; CD antigen CD284; ARMD10; hToll; TLR-4; TOLL; Toll-like receptor 4 |
Application Instructions
| Application Suggestion |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
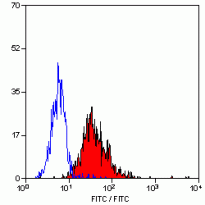
| Application Note | FACS: Use 10 µl of the suggested working dilution to label 10^6 cells or 100 µl whole blood. * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
Properties
| Form | Liquid |
|---|---|
| Purification | Purification with Protein G. |
| Buffer | PBS and 0.09% Sodium azide |
| Preservative | 0.09% Sodium azide |
| Concentration | 1 mg/ml |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | TLR4 |
| Gene Full Name | toll-like receptor 4 |
| Background | The protein encoded by this gene is a member of the Toll-like receptor (TLR) family which plays a fundamental role in pathogen recognition and activation of innate immunity. TLRs are highly conserved from Drosophila to humans and share structural and functional similarities. They recognize pathogen-associated molecular patterns that are expressed on infectious agents, and mediate the production of cytokines necessary for the development of effective immunity. The various TLRs exhibit different patterns of expression. This receptor has been implicated in signal transduction events induced by lipopolysaccharide (LPS) found in most gram-negative bacteria. Mutations in this gene have been associated with differences in LPS responsiveness. Multiple transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Jan 2012] |
| Function | Cooperates with LY96 and CD14 to mediate the innate immune response to bacterial lipopolysaccharide (LPS). Acts via MYD88, TIRAP and TRAF6, leading to NF-kappa-B activation, cytokine secretion and the inflammatory response. Also involved in LPS-independent inflammatory responses triggered by free fatty acids, such as palmitate, and Ni(2+). Responses triggered by Ni(2+) require non-conserved histidines and are, therefore, species-specific. In complex with TLR6, promotes sterile inflammation in monocytes/macrophages in response to oxidized low-density lipoprotein (oxLDL) or amyloid-beta 42. In this context, the initial signal is provided by oxLDL- or amyloid-beta 42-binding to CD36. This event induces the formation of a heterodimer of TLR4 and TLR6, which is rapidly internalized and triggers inflammatory response, leading to the NF-kappa-B-dependent production of CXCL1, CXCL2 and CCL9 cytokines, via MYD88 signaling pathway, and CCL5 cytokine, via TICAM1 signaling pathway, as well as IL1B secretion. [UniProt] |
| Highlight | Related products: TLR4 antibodies; TLR4 ELISA Kits; Anti-Mouse IgG secondary antibodies; Related news: CyTOF-candidate Antibodies Detecting exosomal HMGB1 for ICD research |
| Calculated MW | 96 kDa |
| PTM | N-glycosylated. Glycosylation of Asn-526 and Asn-575 seems to be necessary for the expression of TLR4 on the cell surface and the LPS-response. Likewise, mutants lacking two or more of the other N-glycosylation sites were deficient in interaction with LPS. |
Images (1) Click the Picture to Zoom In