ARG10183
anti-Amyloid Precursor Protein antibody [NT 4A2]
anti-Amyloid Precursor Protein antibody [NT 4A2] for ELISA,IHC-Formalin-fixed paraffin-embedded sections and Human
Neuroscience antibody
Overview
| Product Description | Mouse Monoclonal antibody [NT 4A2] recognizes Amyloid beta peptide |
|---|---|
| Tested Reactivity | Hu |
| Tested Application | ELISA, IHC-P |
| Specificity | This antibody recognizes the N-terminal peptide (DAEFRHDS) of human beta amyloid peptides and also reacts with full length Aβ40, Aβ42 and Aβ43. |
| Host | Mouse |
| Clonality | Monoclonal |
| Clone | NT 4A2 |
| Isotype | IgG1, kappa |
| Target Name | Amyloid Precursor Protein |
| Antigen Species | Human |
| Immunogen | Full length human beta amyloid peptide 42 |
| Epitope | DAEFRHDS |
| Conjugation | Un-conjugated |
| Alternate Names | CVAP; AAA; AICD-50; PN2; 50; Beta-APP42; AID; Gamma-CTF; S-APP-alpha; 57; AD1; PN-II; Beta-APP40; 42; 40; APPI; Alzheimer disease amyloid protein; Amyloid beta A4 protein; PreA4; ABETA; Amyloid intracellular domain 50; CTFgamma; Amyloid intracellular domain 57; 59; AICD-59; S-APP-beta; APP; AICD-57; Amyloid intracellular domain 59; ABPP; Protease nexin-II; Cerebral vascular amyloid peptide |
Application Instructions
| Application Suggestion |
|
||||||
|---|---|---|---|---|---|---|---|
| Application Note | IHC-P: Need heat induced antigen retrieval. ELISA: The antibody can be used as capture antibody in Sandwich ELISA for Aβ42 detection. * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
Properties
| Form | Liquid |
|---|---|
| Purification | Protein G affinity purified |
| Buffer | 0.01M PBS (pH 7.2) |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | APP |
| Gene Full Name | amyloid beta (A4) precursor protein |
| Background | Aβ40, Aβ42 and Aβ43 are different only at the few C-end amino acids. While the C-end amino acids showed no species-specificity in mammalians, the N-terminal amino acid sequences have minor discrepancies between difference species. Amyloid beta peptides Aβ42 and Aβ40 have been investigated extensively for predicating Alzheimer’s disease. A recent study on A Aβ43 in brain showed that Aβ43 is more fibrillogenic than the other amyloid beta peptides and may be useful as a biomarker or therapeutic target for Alzheimer’s disease. Antibody to N-terminal sequence can bind to all the three amyloid beta peptides. |
| Function | Functions as a cell surface receptor and performs physiological functions on the surface of neurons relevant to neurite growth, neuronal adhesion and axonogenesis. Involved in cell mobility and transcription regulation through protein-protein interactions. Can promote transcription activation through binding to APBB1-KAT5 and inhibits Notch signaling through interaction with Numb. Couples to apoptosis-inducing pathways such as those mediated by G(O) and JIP. Inhibits G(o) alpha ATPase activity (By similarity). Acts as a kinesin I membrane receptor, mediating the axonal transport of beta-secretase and presenilin 1. Involved in copper homeostasis/oxidative stress through copper ion reduction. In vitro, copper-metallated APP induces neuronal death directly or is potentiated through Cu(2+)-mediated low-density lipoprotein oxidation. Can regulate neurite outgrowth through binding to components of the extracellular matrix such as heparin and collagen I and IV. The splice isoforms that contain the BPTI domain possess protease inhibitor activity. Induces a AGER-dependent pathway that involves activation of p38 MAPK, resulting in internalization of amyloid-beta peptide and leading to mitochondrial dysfunction in cultured cortical neurons. Provides Cu(2+) ions for GPC1 which are required for release of nitric oxide (NO) and subsequent degradation of the heparan sulfate chains on GPC1. Beta-amyloid peptides are lipophilic metal chelators with metal-reducing activity. Bind transient metals such as copper, zinc and iron. In vitro, can reduce Cu(2+) and Fe(3+) to Cu(+) and Fe(2+), respectively. Beta-amyloid 42 is a more effective reductant than beta-amyloid 40. Beta-amyloid peptides bind to lipoproteins and apolipoproteins E and J in the CSF and to HDL particles in plasma, inhibiting metal-catalyzed oxidation of lipoproteins. Beta-APP42 may activate mononuclear phagocytes in the brain and elicit inflammatory responses. Promotes both tau aggregation and TPK II-mediated phosphorylation. Interaction with Also bind GPC1 in lipid rafts. Appicans elicit adhesion of neural cells to the extracellular matrix and may regulate neurite outgrowth in the brain. The gamma-CTF peptides as well as the caspase-cleaved peptides, including C31, are potent enhancers of neuronal apoptosis. N-APP binds TNFRSF21 triggering caspase activation and degeneration of both neuronal cell bodies (via caspase-3) and axons (via caspase-6). [UniProt] |
| Highlight | Related Antibody Duos and Panels: ARG30063 Beta amyloid peptide 42 ELISA Antibody Duo ARG30064 Beta amyloid peptide 40 ELISA Antibody Duo Related products: Amyloid Precursor Protein antibodies; Amyloid Precursor Protein Duos / Panels; Anti-Mouse IgG secondary antibodies; |
| Research Area | Neuroscience antibody |
| Calculated MW | 87 kDa. (79 - 120 kDa depending on glycosylation level) |
| PTM | Proteolytically processed under normal cellular conditions. Cleavage either by alpha-secretase, beta-secretase or theta-secretase leads to generation and extracellular release of soluble APP peptides, S-APP-alpha and S-APP-beta, and the retention of corresponding membrane-anchored C-terminal fragments, C80, C83 and C99. Subsequent processing of C80 and C83 by gamma-secretase yields P3 peptides. This is the major secretory pathway and is non-amyloidogenic. Alternatively, presenilin/nicastrin-mediated gamma-secretase processing of C99 releases the amyloid beta proteins, amyloid-beta 40 (Abeta40) and amyloid-beta 42 (Abeta42), major components of amyloid plaques, and the cytotoxic C-terminal fragments, gamma-CTF(50), gamma-CTF(57) and gamma-CTF(59). Many other minor beta-amyloid peptides, beta-amyloid 1-X peptides, are found in cerebral spinal fluid (CSF) including the beta-amyloid X-15 peptides, produced from the cleavage by alpha-secretase and all terminating at Gln-686. Proteolytically cleaved by caspases during neuronal apoptosis. Cleavage at Asp-739 by either caspase-6, -8 or -9 results in the production of the neurotoxic C31 peptide and the increased production of beta-amyloid peptides. N- and O-glycosylated. O-glycosylation on Ser and Thr residues with core 1 or possibly core 8 glycans. Partial tyrosine glycosylation (Tyr-681) is found on some minor, short beta-amyloid peptides (beta-amyloid 1-15, 1-16, 1-17, 1-18, 1-19 and 1-20) but not found on beta-amyloid 38, beta-amyloid 40 nor on beta-amyloid 42. Modification on a tyrosine is unusual and is more prevelant in AD patients. Glycans had Neu5AcHex(Neu5Ac)HexNAc-O-Tyr, Neu5AcNeu5AcHex(Neu5Ac)HexNAc-O-Tyr and O-AcNeu5AcNeu5AcHex(Neu5Ac)HexNAc-O-Tyr structures, where O-Ac is O-acetylation of Neu5Ac. Neu5AcNeu5Ac is most likely Neu5Ac 2,8Neu5Ac linked. O-glycosylations in the vicinity of the cleavage sites may influence the proteolytic processing. Appicans are L-APP isoforms with O-linked chondroitin sulfate. Phosphorylation in the C-terminal on tyrosine, threonine and serine residues is neuron-specific. Phosphorylation can affect APP processing, neuronal differentiation and interaction with other proteins. Phosphorylated on Thr-743 in neuronal cells by Cdc5 kinase and Mapk10, in dividing cells by Cdc2 kinase in a cell-cycle dependent manner with maximal levels at the G2/M phase and, in vitro, by GSK-3-beta. The Thr-743 phosphorylated form causes a conformational change which reduces binding of Fe65 family members. Phosphorylation on Tyr-757 is required for SHC binding. Phosphorylated in the extracellular domain by casein kinases on both soluble and membrane-bound APP. This phosphorylation is inhibited by heparin. Extracellular binding and reduction of copper, results in a corresponding oxidation of Cys-144 and Cys-158, and the formation of a disulfide bond. In vitro, the APP-Cu(+) complex in the presence of hydrogen peroxide results in an increased production of beta-amyloid-containing peptides. Trophic-factor deprivation triggers the cleavage of surface APP by beta-secretase to release sAPP-beta which is further cleaved to release an N-terminal fragment of APP (N-APP). Beta-amyloid peptides are degraded by IDE. |
Images (1) Click the Picture to Zoom In
-
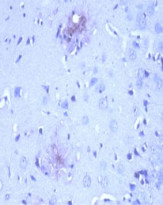
ARG10183 anti-Amyloid Precursor Protein antibody [NT 4A2] IHC-P image
Immunohistochemistry: After heat-induced antigen retrieval, Formalin-fixed and paraffin-embedded brain tissue section from 12 month old APPswe/PSEN1dE9 transgenic Alzheimer’s disease mouse model stained with ARG10183 anti-Amyloid Precursor Protein antibody [NT 4A2] at 10 µg/ml, 4°C and overnight.