ARG54672
anti-Amyloid Precursor Protein antibody
anti-Amyloid Precursor Protein antibody for ELISA,ICC/IF,IHC-Formalin-fixed paraffin-embedded sections,Western blot and Human,Mouse,Rat
Neuroscience antibody
Overview
| Product Description | Rabbit Polyclonal antibody recognizes Amyloid Precursor Protein |
|---|---|
| Tested Reactivity | Hu, Ms, Rat |
| Tested Application | ELISA, ICC/IF, IHC-P, WB |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Target Name | Amyloid Precursor Protein |
| Immunogen | Synthetic peptide (18 aa) within aa. 180-230 of Human APP protein. |
| Conjugation | Un-conjugated |
| Alternate Names | CVAP; AAA; AICD-50; PN2; 50; Beta-APP42; AID; Gamma-CTF; S-APP-alpha; 57; AD1; PN-II; Beta-APP40; 42; 40; APPI; Alzheimer disease amyloid protein; Amyloid beta A4 protein; PreA4; ABETA; Amyloid intracellular domain 50; CTFgamma; Amyloid intracellular domain 57; 59; AICD-59; S-APP-beta; APP; AICD-57; Amyloid intracellular domain 59; ABPP; Protease nexin-II; Cerebral vascular amyloid peptide |
Application Instructions
| Application Suggestion |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Application Note | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. | ||||||||||
| Positive Control | Rat Brain Tissue Lysate |
Properties
| Form | Liquid |
|---|---|
| Purification | Affinity purification with immunogen. |
| Buffer | PBS and 0.02% Sodium azide |
| Preservative | 0.02% Sodium azide |
| Concentration | 1 mg/ml |
| Storage Instruction | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Database Links | |
|---|---|
| Gene Symbol | APP |
| Gene Full Name | amyloid beta (A4) precursor protein |
| Background | APP Antibody: Accumulation of the amyloid-beta peptide (Abeta) in the cerebral cortex is a critical event in the pathogenesis of Alzheimer's disease. The beta-amyloid protein precursor (APP) is cleaved by one of two beta-secretases (BACE and BACE2), producing a soluble derivative of the protein and a membrane anchored 99-amino acid carboxy-terminal fragment (C99). The C99 fragment serves as substrate for gamma-secretase to generate the 4 kDa amyloid-beta peptide (Abeta), which is deposited in the Alzheimer's disease patient's brains. Recently, Death Receptor 6 (DR6) was found to interact with an amino-terminal fragment of the Beta-amyloid protein (N-APP) in neurons, activating a caspase 6-dependent apoptotic event leading to axonal degeneration and pruning during development, suggesting that these two proteins are involved in neural development and may possibly play a role in Alzheimer's disease. | |
| Research Area | Neuroscience antibody |
| Calculated MW | 87 kDa. (79 - 120 kDa depending on glycosylation level) |
| PTM | Proteolytically processed under normal cellular conditions. Cleavage either by alpha-secretase, beta-secretase or theta-secretase leads to generation and extracellular release of soluble APP peptides, S-APP-alpha and S-APP-beta, and the retention of corresponding membrane-anchored C-terminal fragments, C80, C83 and C99. Subsequent processing of C80 and C83 by gamma-secretase yields P3 peptides. This is the major secretory pathway and is non-amyloidogenic. Alternatively, presenilin/nicastrin-mediated gamma-secretase processing of C99 releases the amyloid beta proteins, amyloid-beta 40 (Abeta40) and amyloid-beta 42 (Abeta42), major components of amyloid plaques, and the cytotoxic C-terminal fragments, gamma-CTF(50), gamma-CTF(57) and gamma-CTF(59). Many other minor beta-amyloid peptides, beta-amyloid 1-X peptides, are found in cerebral spinal fluid (CSF) including the beta-amyloid X-15 peptides, produced from the cleavage by alpha-secretase and all terminating at Gln-686. Proteolytically cleaved by caspases during neuronal apoptosis. Cleavage at Asp-739 by either caspase-6, -8 or -9 results in the production of the neurotoxic C31 peptide and the increased production of beta-amyloid peptides. N- and O-glycosylated. O-glycosylation on Ser and Thr residues with core 1 or possibly core 8 glycans. Partial tyrosine glycosylation (Tyr-681) is found on some minor, short beta-amyloid peptides (beta-amyloid 1-15, 1-16, 1-17, 1-18, 1-19 and 1-20) but not found on beta-amyloid 38, beta-amyloid 40 nor on beta-amyloid 42. Modification on a tyrosine is unusual and is more prevelant in AD patients. Glycans had Neu5AcHex(Neu5Ac)HexNAc-O-Tyr, Neu5AcNeu5AcHex(Neu5Ac)HexNAc-O-Tyr and O-AcNeu5AcNeu5AcHex(Neu5Ac)HexNAc-O-Tyr structures, where O-Ac is O-acetylation of Neu5Ac. Neu5AcNeu5Ac is most likely Neu5Ac 2,8Neu5Ac linked. O-glycosylations in the vicinity of the cleavage sites may influence the proteolytic processing. Appicans are L-APP isoforms with O-linked chondroitin sulfate. Phosphorylation in the C-terminal on tyrosine, threonine and serine residues is neuron-specific. Phosphorylation can affect APP processing, neuronal differentiation and interaction with other proteins. Phosphorylated on Thr-743 in neuronal cells by Cdc5 kinase and Mapk10, in dividing cells by Cdc2 kinase in a cell-cycle dependent manner with maximal levels at the G2/M phase and, in vitro, by GSK-3-beta. The Thr-743 phosphorylated form causes a conformational change which reduces binding of Fe65 family members. Phosphorylation on Tyr-757 is required for SHC binding. Phosphorylated in the extracellular domain by casein kinases on both soluble and membrane-bound APP. This phosphorylation is inhibited by heparin. Extracellular binding and reduction of copper, results in a corresponding oxidation of Cys-144 and Cys-158, and the formation of a disulfide bond. In vitro, the APP-Cu(+) complex in the presence of hydrogen peroxide results in an increased production of beta-amyloid-containing peptides. Trophic-factor deprivation triggers the cleavage of surface APP by beta-secretase to release sAPP-beta which is further cleaved to release an N-terminal fragment of APP (N-APP). Beta-amyloid peptides are degraded by IDE. |
Images (3) Click the Picture to Zoom In
-
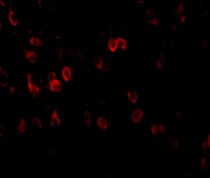
ARG54672 anti-APP antibody ICC/IF image
Immunofluorescence: mouse brain tissue stained with ARG54672 anti-APP antibody at 20 μg/ml.
-
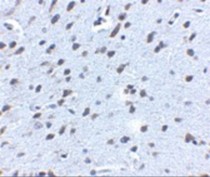
ARG54672 anti-APP antibody IHC image
Immunohistochemistry: mouse brain tissue stained with ARG54672 anti-APP antibody at 2.5 μg/ml.
-
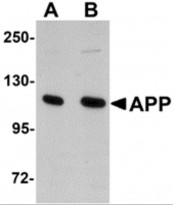
ARG54672 anti-APP antibody WB image
Western blot: rat brain tissue lysate stained with ARG54672 anti-APP antibody at (A) 1 and (B) 2 μg/ml.