ARG70317
Human PCSK9 recombinant protein (His-tagged, C-ter)
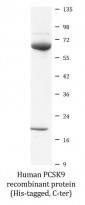
Human PCSK9 recombinant protein (His-tagged, C-ter) for Binding Activity,SDS-PAGE and Human
Overview
| Product Description | HEK293 expressed, His-tagged (C-ter) Human PCSK9 recombinant protein. |
|---|---|
| Tested Reactivity | Hu |
| Tested Application | Binding, SDS-PAGE |
| Target Name | PCSK9 |
| Species | Human |
| A.A. Sequence | Gln31 - Gln692 of Human PCSK9 (NP_777596.2) with 6X His tag at the C - terminus. |
| Expression System | HEK293 |
| Alternate Names | PC9; Subtilisin/kexin-like protease PC9; Proprotein convertase 9; Proprotein convertase subtilisin/kexin type 9; Neural apoptosis-regulated convertase 1; FH3; EC 3.4.21.-; HCHOLA3; NARC1; LDLCQ1; NARC-1 |
Application Instructions
| Application Note | Binding activity test: Measured by its binding ability in a functional ELISA. Immobilized Human LDL-R at 3 ug/ml (100 µl/well) can bind Human PCSK9 with a linear range of 0.5-2ug/ml. |
|---|
Properties
| Form | Powder |
|---|---|
| Purification Note | 0.22 µm filter sterilized. Endotoxin level is <0.1 EU/µg of the protein, as determined by the LAL test. |
| Purity | > 92% (by SDS-PAGE) |
| Buffer | PBS (pH 7.4) |
| Reconstitution | Reconstitute to a concentration of 0.1 - 0.5 mg/ml in sterile distilled water. |
| Storage Instruction | For long term, lyophilized protein should be stored at -20°C or -80°C. After reconstitution, aliquot and store at -20°C for up to one month, at 2-8°C for up to one week. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Gene Symbol | PCSK9 |
|---|---|
| Gene Full Name | proprotein convertase subtilisin/kexin type 9 |
| Background | This gene encodes a member of the subtilisin-like proprotein convertase family, which includes proteases that process protein and peptide precursors trafficking through regulated or constitutive branches of the secretory pathway. The encoded protein undergoes an autocatalytic processing event with its prosegment in the ER and is constitutively secreted as an inactive protease into the extracellular matrix and trans-Golgi network. It is expressed in liver, intestine and kidney tissues and escorts specific receptors for lysosomal degradation. It plays a role in cholesterol and fatty acid metabolism. Mutations in this gene have been associated with autosomal dominant familial hypercholesterolemia. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Feb 2014] |
| Function | Crucial player in the regulation of plasma cholesterol homeostasis. Binds to low-density lipid receptor family members: low density lipoprotein receptor (LDLR), very low density lipoprotein receptor (VLDLR), apolipoprotein E receptor (LRP1/APOER) and apolipoprotein receptor 2 (LRP8/APOER2), and promotes their degradation in intracellular acidic compartments (PubMed:18039658). Acts via a non-proteolytic mechanism to enhance the degradation of the hepatic LDLR through a clathrin LDLRAP1/ARH-mediated pathway. May prevent the recycling of LDLR from endosomes to the cell surface or direct it to lysosomes for degradation. Can induce ubiquitination of LDLR leading to its subsequent degradation (PubMed:18799458, PubMed:17461796, PubMed:18197702, PubMed:22074827). Inhibits intracellular degradation of APOB via the autophagosome/lysosome pathway in a LDLR-independent manner. Involved in the disposal of non-acetylated intermediates of BACE1 in the early secretory pathway (PubMed:18660751). Inhibits epithelial Na(+) channel (ENaC)-mediated Na(+) absorption by reducing ENaC surface expression primarily by increasing its proteasomal degradation. Regulates neuronal apoptosis via modulation of LRP8/APOER2 levels and related anti-apoptotic signaling pathways. [UniProt] |
| Cellular Localization | Cytoplasm. Secreted. Endosome. Lysosome. Cell surface. ER. Golgi apparatus. Note=Autocatalytic cleavage is required to transport it from the endoplasmic reticulum to the Golgi apparatus and for the secretion of the mature protein. Localizes to the endoplasmic reticulum in the absence of LDLR and colocalizes to the cell surface and to the endosomes/lysosomes in the presence of LDLR. The sorting to the cell surface and endosomes is required in order to fully promote LDLR degradation. [UniProt] |
| Calculated MW | 74 kDa |
| PTM | Cleavage by furin and PCSK5 generates a truncated inactive protein that is unable to induce LDLR degradation. Undergoes autocatalytic cleavage in the endoplasmic reticulum to release the propeptide from the N-terminus and the cleavage of the propeptide is strictly required for its maturation and activation. The cleaved propeptide however remains associated with the catalytic domain through non-covalent interactions, preventing potential substrates from accessing its active site. As a result, it is secreted from cells as a propeptide-containing, enzymatically inactive protein. Phosphorylation protects the propeptide against proteolysis. [UniProt] |
Images (1) Click the Picture to Zoom In