ARG70280
Human MMP13 recombinant protein (Active) (His-tagged, C-ter)
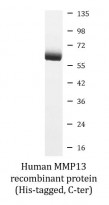
Human MMP13 recombinant protein (Active) (His-tagged, C-ter) for Functional study,SDS-PAGE and Human
Overview
| Product Description | HEK293 expressed, His-tagged (C-ter) Active Human MMP13 recombinant protein. |
|---|---|
| Tested Reactivity | Hu |
| Tested Application | FuncSt, SDS-PAGE |
| Target Name | MMP13 |
| Species | Human |
| A.A. Sequence | Leu20 - Cys471 of Human MMP13 (NP_002418.1) with 6X His tag at the C - terminus. |
| Expression System | HEK293 |
| Activity | Active |
| Activity Note | Measured in a cell migration assay using A549 cells. 10 ng/ml of Recombinant Human MMP-13 can effectively induce A549 cells migration. |
| Alternate Names | CLG3; EC 3.4.24.-; MANDP1; MMP-13; Collagenase 3; Matrix metalloproteinase-13 |
Properties
| Form | Powder |
|---|---|
| Purification Note | 0.22 µm filter sterilized. Endotoxin level is <0.1 EU/µg of the protein, as determined by the LAL test. |
| Purity | > 95% (by SDS-PAGE) |
| Buffer | PBS (pH 7.4) |
| Reconstitution | Reconstitute to a concentration of 0.1 - 0.5 mg/ml in sterile distilled water. |
| Storage Instruction | For long term, lyophilized protein should be stored at -20°C or -80°C. After reconstitution, aliquot and store at -20°C for up to one month, at 2-8°C for up to one week. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. |
| Note | For laboratory research only, not for drug, diagnostic or other use. |
Bioinformation
| Gene Symbol | MMP13 |
|---|---|
| Gene Full Name | matrix metallopeptidase 13 |
| Background | This gene encodes a member of the peptidase M10 family of matrix metalloproteinases (MMPs). Proteins in this family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. The encoded preproprotein is proteolytically processed to generate the mature protease. This protease cleaves type II collagen more efficiently than types I and III. It may be involved in articular cartilage turnover and cartilage pathophysiology associated with osteoarthritis. Mutations in this gene are associated with metaphyseal anadysplasia. This gene is part of a cluster of MMP genes on chromosome 11. [provided by RefSeq, Jan 2016] |
| Function | Plays a role in the degradation of extracellular matrix proteins including fibrillar collagen, fibronectin, TNC and ACAN. Cleaves triple helical collagens, including type I, type II and type III collagen, but has the highest activity with soluble type II collagen. Can also degrade collagen type IV, type XIV and type X. May also function by activating or degrading key regulatory proteins, such as TGFB1 and CCN2. Plays a role in wound healing, tissue remodeling, cartilage degradation, bone development, bone mineralization and ossification. Required for normal embryonic bone development and ossification. Plays a role in the healing of bone fractures via endochondral ossification. Plays a role in wound healing, probably by a mechanism that involves proteolytic activation of TGFB1 and degradation of CCN2. Plays a role in keratinocyte migration during wound healing. May play a role in cell migration and in tumor cell invasion. [UniProt] |
| Cellular Localization | Secreted, extracellular space, extracellular matrix. Secreted. [UniProt] |
| Calculated MW | 54 kDa |
| PTM | The proenzyme is activated by removal of the propeptide; this cleavage can be effected by other matrix metalloproteinases, such as MMP2, MMP3 and MMP14 and may involve several cleavage steps. Cleavage can also be autocatalytic, after partial maturation by another protease or after treatment with 4-aminophenylmercuric acetate (APMA) (in vitro). N-glycosylated. Tyrosine phosphorylated by PKDCC/VLK. [UniProt] |
Images (1) Click the Picture to Zoom In