Quality control for engineered T cell therapy
Quality control for engineered T cell therapy
Engineered T cell therapy, such as CAR-T and TCR-T, is designed to redirect a patient's T cells to specifically target and destroy tumor cells. This promising therapy is based on genetic engineering by viral transduction of chimeric antigen receptor (CAR) or transgenic TCR genes into T cells. The preparation of transgene DNA requires multiple hosts, and the viral transduction may lead to high copy number transgene insertion in T cells. Quality control is critical to ensure the safety of engineered T cell therapy. arigo offers a series of kits to evaluate the purity, potency, and safety of therapeutic T cells.
|
|
● Quality control of transgene DNA
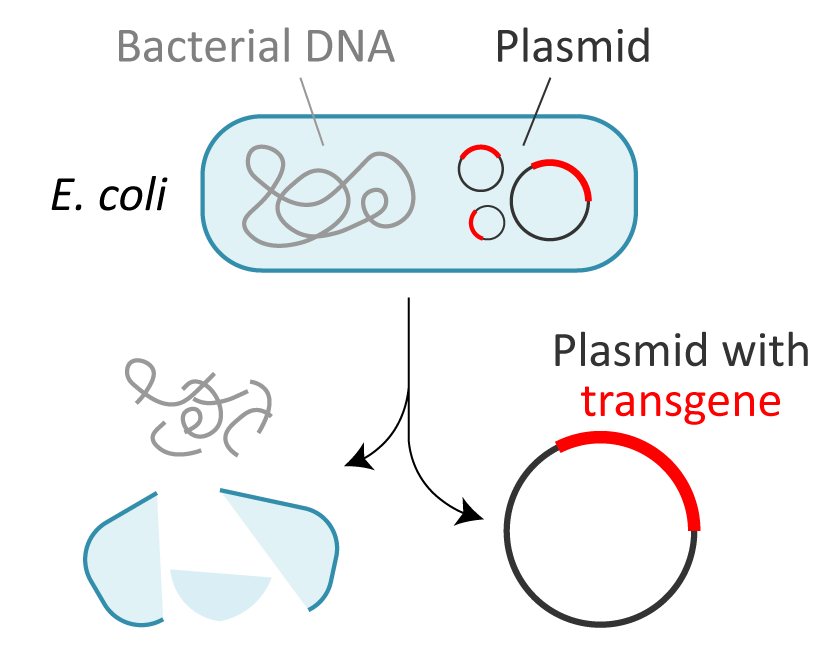 |
The transgene DNA is usually amplified by E. coli. It is important for the safety of biopharmaceuticals to ensure there is no contamination of E. coli contents, including nucleic acids and proteins. |
|
|
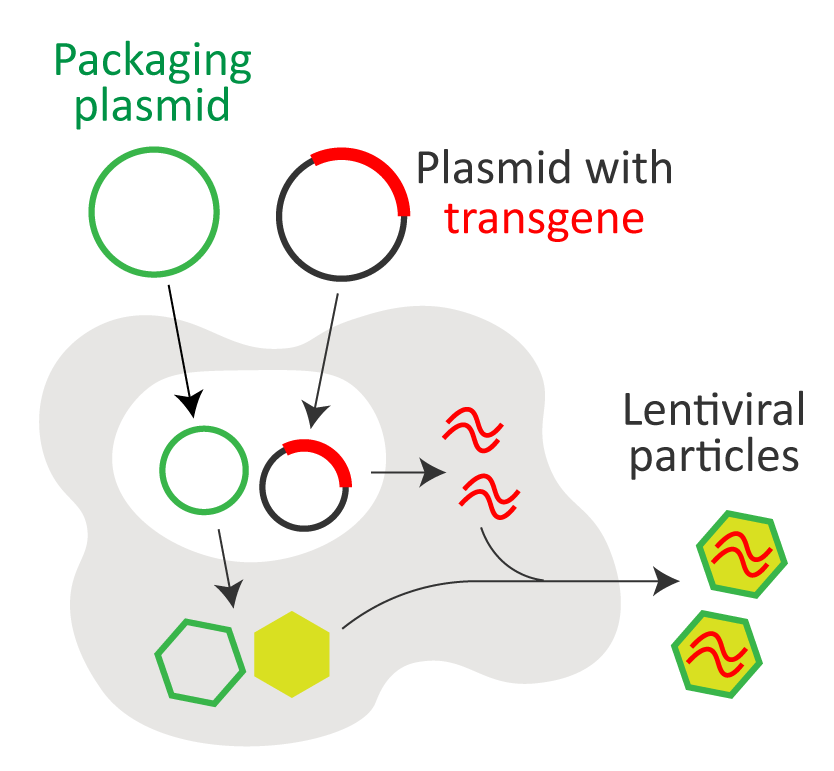
● Quality control of lentiviral packaging
| HIV-1-based lentiviral vector is frequently used to stably deliver transgene into therapeutic T cells. Mammalian 293T cells are the most common host for lentivirus packaging and production. arigo offers lentivirus quantitation kit as well as multiple detection kits to ensure there is no residual host contents or solutions used for preparation of lentivirus particles. |
|
|
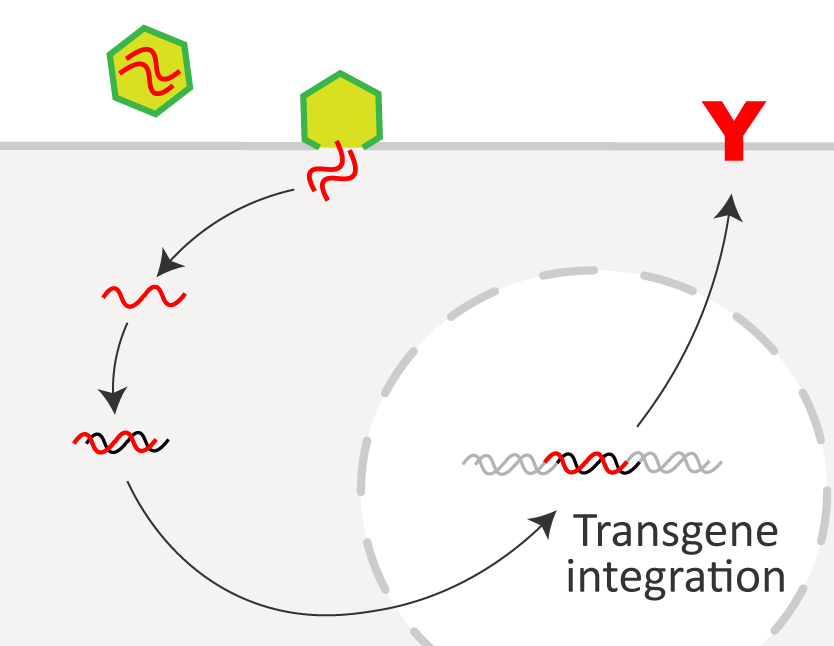
● Quality control of engineered T cells
| Lentiviral transduction has been used extensively due to the ability to carry large transgene loads, efficiently integrate into the host genome, and allow for long-term transgene expression. However, lentiviral vector also presents risk of insertional mutagenesis. Quality control to check the integrated lentiviral vector copy number, mycoplasma contamination, and cytotoxicity toward target cells is essential for the release of biopharmaceuticals. |
 |