Stem cell and the regenerative medicine: Ready for the patients?
Stem cell and the regenerative medicine: Ready for the patients?
|
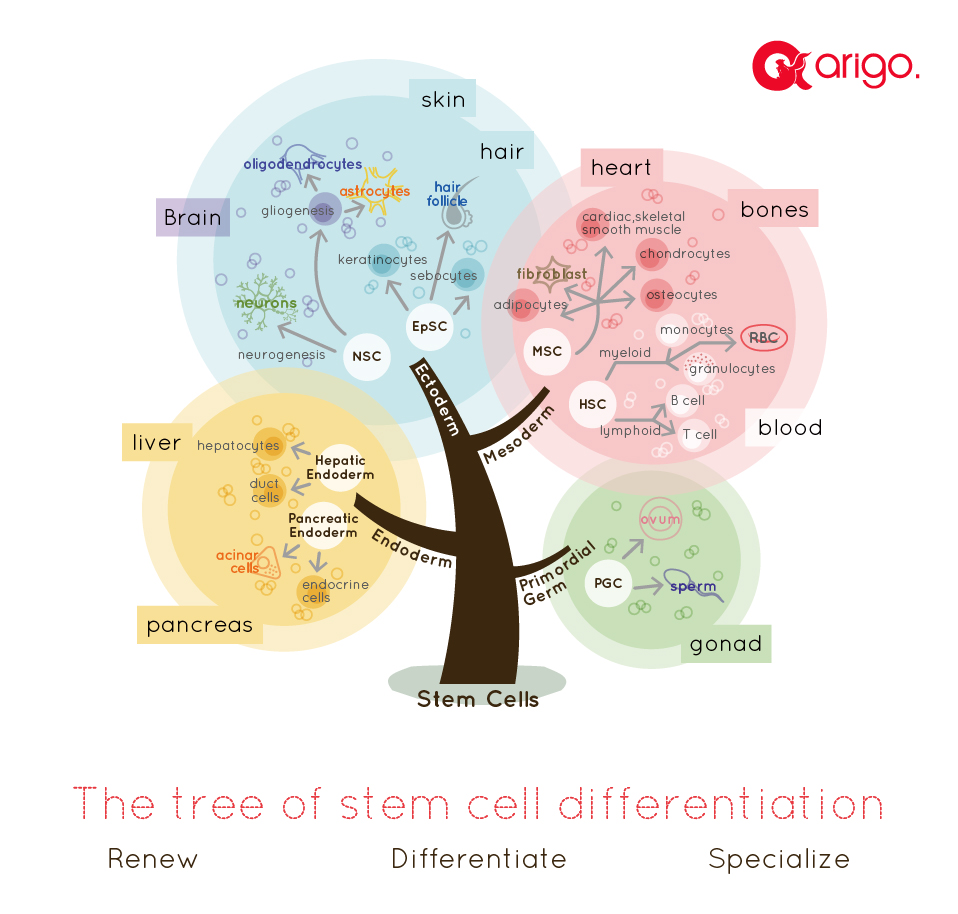
MSC: Mesenchymal stem cells, HSC: Hematopoietic stem cells,
Stem cells Stem cells are able to self-renew, indefinitely dividing and generating exact copies of themselves. Under the right condition, they can be differentiated virtually into any kind of specialized cell progeny which are able to function properly or replace wounded tissues in our body. Stem cells come in different types and shapes, each play a distinct role in our body as we grow and develop. Although some exist in a narrow window during development, most others are found in specific part of the body during our lifetime. Generally, stem cells can be categorized into three different groups: 1. Embryonic stem cells (ESCs): A totipotent population extracted from embryo which are able to differentiate into all specialized cell types in our body. 2. Somatic stem cells: Multipotent or unipotent population that are only able to give rise to limited lineage of specialized cells. 3. Induced pluripotent stem cells (iPSCs): Pluripotent cell population generated by artificially inserting several genes into somatic cells.
Stem cell differentiation The process of stem cell differentiation is a highly organized and unidirectional process. A totipotent stem cell is able to different into 3 different germ layers (ectoderm, endoderm, mesoderm) and primordial germ cells. Multipotent stem cells are then differentiated from these germ layers in an orderly manner. For example, ectoderm makes neural stem cells and epidermal stem cells; endoderm produces hepatic and pancreatic stem cells; mesenchymal and hematopoietic stem cells are developed form the mesoderm. Given the high degree of plasticity, scientists have been putting extra effort in understanding the mechanism of stem cell differentiation, so that the process of directed differentiation can be optimized in order to give rise to usable materials in stem cell therapy.
Regenerative medicine research Science and technology advancement are bringing regenerative medicine closer to reality. We constantly hear about breakthroughs that provide promising alternatives to combat injuries, diseases and aging. For example, offspring from oocytes generated by ESCs or iPSCs was successfully born, offering hopes for curing infertility (Hayashi et al). In a recent report, scientists found a way to make red blood cells by knocking out SH2B3 gene in Hematopoietic Stem Cells using CRISPR/Cas9 genome editing, potentially putting an end to short supply in blood banks (Giani et al). These news are exciting, but why are stem cells therapies not widely implemented yet? The process of drug development is long and technically demanding to ensure safety and effectiveness of these drugs before they are approved in clinical trials. Generally, it takes 15 to 20 years before a new treatment is made available in the clinic. In fact, most ideas do not even become approved treatments. Nonetheless, researchers continue to hinge on learning the fundamentals of biological process in view of inventing better regenerative medicine for patients. These include better understanding the cause and progression of diseases, creating new technologies to accelerate drug discovery, and developing creative methods to enhance directed differentiation of stem cells.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Differentiation Marker
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||